Supplemental Material for Mangale et al., Science 2008 (in press)
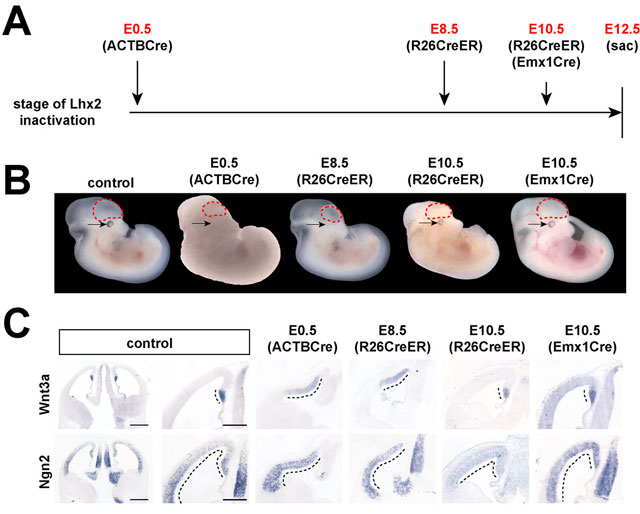
Defining the E8.5-E10.5 critical period of Lhx2 selector activity. (A) Timeline of Lhx2 inactivation by different Cre drivers. Lhx2 is inactivated starting from: E0.5 [ACTBCre, (Lewandoski et al., 1997)]; E8.5 [R26CreER, (Badea et al., 2003)/tamoxifen injection at E8.5]; E10.5 [R26CreER, (Badea et al., 2003)/tamoxifen injection at E10.5; or Emx1-Cre (Jin et al., 2000)]. (B) Lateral views of E12.5 control and mutant embryos. E0.5 and E8.5 Lhx2 inactivation produce similar gross phenotypes. E10.5 Lhx2 inactivation results in subtle defects in the eye (arrow) and possibly in the telencephalon (dotted lines). Montage created using separate embryo images against a uniform background for visual clarity. (C) In situ hybridization of adjacent coronal sections of E12.5 control and mutant embryos, boxed regions enlarged (right). Hem expansion (Wnt3a) is similar following E0.5 or E8.5 inactivations, but subtle after E10.5 inactivation. Dorsal telencephalic loss (Ngn2) is also less severe with delayed Lhx2 inactivation, indicating a temporal gradient in Lhx2 selector functions. Dotted lines designate expression domains. Scale bars: low magnification, 500 mm; high magnification, 300 mm.
===========================================================================================
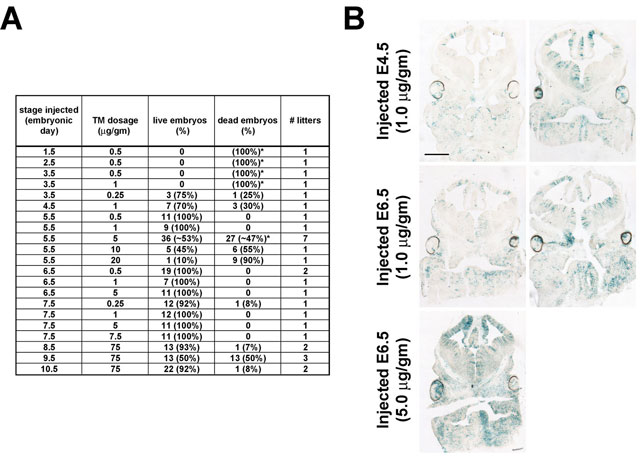
Tamoxifen dosage and timing optimizations. (A) Table of embryonic stage of embryos receiving tamoxifen (TM) via maternal intraperitioneal injection, dosage of TM, number and percentage of viable versus nonviable embryos, and the number of litters examined. Introduction of TM prior to embryo implantation into the uterine wall at E4.5 appeared to be toxic at dosages as low as 0.25 mg/gm. Embryonic toxicity resulting from TM injections between E5.5-E7.5 appeared to be dose dependent. Higher dosages (75 mg/gm) injected from E8.5-E10.5 resulted in relatively low toxicity, corresponding to previous reports (Hayashi and McMahon, 2002). Asterisks designate injections causing complete toxicity to some or all litters examined (embryo numbers undetermined or estimated by sac number). (B) Xgal staining (blue) of E12.5 R26CreER/fl (Badea et al., 2003; Soriano, 1999) coronal sections after injection with: 1 mg/gm TM on E4.5; 1 mg/gm TM on E6.5 or 5 mg/gm TM on E6.5. 1 mg/gm TM dose resulted in greater variability in recombination frequency (compare right versus left panels for the 1 mg/gm dosages), whereas 5 mg/gm TM resulted in more consistent levels of recombination between littermates.
===========================================================================================
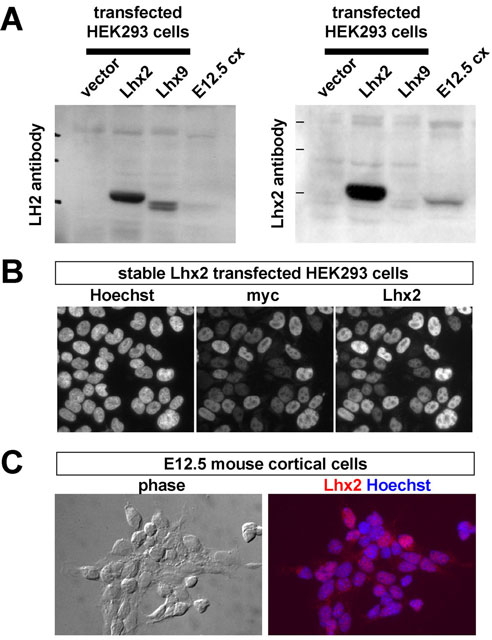
Characterization and validation of an Lhx2-specific antibody. (A) Western blots of homogenates prepared from HEK293 cells, stably transfected with either myc-tagged Lhx2 or myc-tagged Lhx9, and E12.5 cortex. The LH2 antibody (Liem et al., 1997) (left) recognized both Lhx2 and Lhx9, while the crude Lhx2 antipeptide antiserum (right) recognized Lhx2, but not Lhx9. The Lhx2 antiserum also yielded a distinct band in E12.5 cortical homogenates. (B) Immunocytochemistry of HEK293 cells stably transfected with myc-tagged Lhx2, using anti-myc or the Lhx2-specific antibody after affinity purification. Note the nuclear localization and similar relative myc and Lhx2 intensities. (C) Immunocytochemistry of acutely-dissociated E12.5 wild-type cortical cells using the affinity-purified Lhx2 antibody (red).
===========================================================================================
References
Badea, T. C., Wang, Y. and Nathans, J. (2003). A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci 23, 2314-22.
Hayashi, S. and McMahon, A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244, 305-18.
Jin, X. L., Guo, H., Mao, C., Atkins, N., Wang, H., Avasthi, P. P., Tu, Y. T. and Li, Y. (2000). Emx1-specific expression of foreign genes using "knock-in" approach. Biochem Biophys Res Commun 270, 978-82.
Lewandoski, M., Meyers, E. N. and Martin, G. R. (1997). Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol 62, 159-68.
Liem, K. F., Jr., Tremml, G. and Jessell, T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127-38.
Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21, 70-1.